
Coulombic Efficiency (CE) is the key metric used to compare and rank cells and Coulombic Inefficiency per hour (CIE/hr) is an extension of CE that allows comparison of cells cycled at both different rates and within different voltage intervals. These are invaluable tools that help us make informed decisions on cell chemistries on a per-application basis.
Since CIE/hr is obtained by normalizing CE for time, the underlying mechanisms that govern both are the same. For that reason, it’s well worth it to dig deeper into the ingredients that make up CE. Which brings us to the first of two components that make up the CE of a cell: capacity fade.
What is Capacity Fade?
As batteries are cycled and age, the available lithium inventory irreversibly decreases. Lithium depletes for a variety of reasons which themselves are affected by things like time, cycle number, temperature, operational voltage, chemistry, etc. The loss rate of accessible lithium is known as capacity fade.
Have you ever kept maple syrup in a glass jar before? If you have, you might have noticed some crystallization in the bottom of the jar. When that jar was initially filled there was no crystallization but, if used daily, by the time the jar is empty, a small number of crystals will have formed. Those crystals prevent you from getting all the maple syrup out of the jar that was first put in. You now refill the jar and realize that you cannot put quite as much in as the first time you filled it. And again, by the time the jar is empty, even more crystals have formed, and even more maple syrup was forever lost to crystallization.
The more the jar is emptied and refilled, and the longer the jar sits in the fridge, the more crystals form and the less space there is to store the syrup. The reduction in jar volume available to be filled with maple syrup is analogous to the irreversible loss of capacity in a Li-ion cell.
Why Li-Ion Cells Lose Capacity
There are lots of ways that Li-ion cells can lose capacity over time. Perhaps the most prominent way that happens is that electrolyte components can react at the surface of the negative electrode and lead to the formation of a solid electrolyte interface. For example, a solvent reduction reaction occurs when a Li-ion, solvent molecule, and electron react at the negative electrode surface. This reaction is irreversible and reduces the amount of available lithium that can be inserted and removed from the electrodes during cycling, reducing the cell capacity.
This can be partially fixed with electrolyte additives that help reduce the rate of these reactions by improving the protective layer on the surface of the negative electrode. In addition to various electrochemical mechanisms, it’s also possible for some of the electrode materials to become “inactive” due to particle cracking or loss of electrical contact from the current collectors, leading to “dead” material and even less available lithium. Irreversible phase transformations of electrode materials can also “lock” lithium in place, further reducing the cell capacity.
Taken together, the loss of available lithium for charge and discharge is referred to as lithium “inventory” loss. The degree with which the discharge capacity of a cell diminishes over time indicates how much lithium is irreversibly lost due to reactions with the electrolyte and active electrode material loss; this is called capacity fade. Determining the contribution of each mechanism towards the capacity fade can be made clearer through “dV/dQ analysis”, with very precise measurements offered by UHPC.
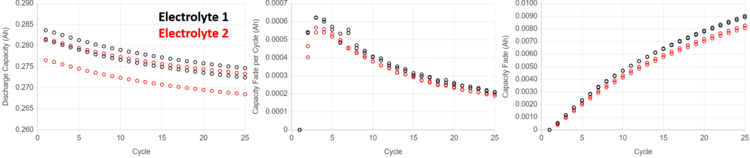
The figure above demonstrates a real case study where differences in capacity fade for nearly identical cells are detected after only 25 cycles on UHPC tests. Two sets of pair cells are shown where the only difference between them is the choice of electrolyte additive; electrolyte 1 (black) contains no additives, while electrolyte 2 (red) contains 1% of some additive. The discharge capacity versus cycle number (far-left panel) can be re-plotted in two ways that elucidate capacity fade: the middle panel shows the capacity loss each cycle, and the far-right panel shows the cumulative capacity loss as a function of cycle number.
After only 25 cycles, cells with 1% additive (electrolyte 2), have lost ~0.001 Ah less than cells with electrolyte 1. Since both the positive and negative electrodes in these cells are the same, it can be deduced that electrolyte 2 leads to fewer irreversible reactions that consume lithium (less “lithium inventory loss”). It is important to note that all of these cells were cycled at the same rate. However, the concepts highlighted in our previous post can be applied analogously to account for cells cycled at different rates. Detecting differences in lithium inventory loss between different cell chemistries in early cycle life is invaluable for quickly screening how electrolyte-electrode combinations influence lithium inventory loss.
